Abstract
Biocompatible functional nanomaterials, when integrated into living systems, have the potential to both augment basic biological functions and introduce completely new functions into organisms. Incorporating functional nanomaterials with unique physical properties into living cells has created a new paradigm in synthetic biology, allowing researchers to manipulate and even enhance biology in ways not possible with traditional chemical or genetic modifications. In this review, first, we review the latest developments in interfacing synthetic nanomaterials with organisms at the cellular level, and relevant applications, especially to neuromodulation and augmented photosynthesis. Then, we highlight the need for targeting nanomaterials to specific cells or subcellular destinations within large, multicellular organisms in order to achieve precise control over these systems in a biocompatible manner. In particular, we discuss recent advances in in vivo nanomaterial synthesis and how they can be used to achieve this precise nanomaterial integration. Finally, we introduce genetically-targetable chemical assembly for in situ nanomaterial synthesis as an emerging tool. We discuss the perspectives of novel cell-type-specific biological manipulations by these genetically-targeted methods.
Export citation and abstract BibTeX RIS
1. Introduction
Synthetic biology seeks to engineer biological systems with novel functions, either by building entirely new biological systems in a bottom-up approach, or by modifying existing biology [1–7]. Due to the inherent complexity of biological systems, creating an entire synthetic biological system from the bottom-up remains challenging [6, 7]. Here, we focus on recent advances in the top-down approach that modify existing biology to meet current needs. Historically, genetic and metabolic engineering have been the most commonly used tools for modifying and re-working existing living systems [1–4]. However, these methods remain inherently limited by the physical properties of biosynthesized materials. The incorporation of nanomaterials with diverse functions into living organisms to create nanobiohybrids can overcome many of these limitations. Recent advances in nanotechnology have enabled the incorporation of a variety of functional nanomaterials with unique chemical, physical, magnetic, and electronic properties into a wide range of single-cell and multicellular organisms. In particular, the seamless integration of electronically functional nanomaterials and devices with biology has introduced naturally non-existing functions into a range of living systems. As such, nanomaterials have become an increasingly important part of the synthetic biology toolbox [8, 9].
Most of the efforts in this emerging field have focused on interfacing living systems with synthetically produced nanomaterials (figure 1(a)). These early successes have demonstrated that incorporating conductive nanomaterials into cells and tissues can modulate the electrophysiology of electroactive tissues, including the heart and nervous systems, which has led to the enhancement of synaptic activity [10], neuron firing [11], and synchronic contractions of cardiac cells [12], in addition to enabling optoelectronic control of action potentials [13–15]. Likewise, the use of semiconducting materials such as single-walled carbon nanotubes (SWCNTs) [16], InP nanoparticles [9], and gold nanoclusters (AuNCs) [17] to enhance photosynthesis or even introduce it into nonphotosynthetic organisms represents another promising application of nano-enabled synthetic biology. Although these examples demonstrate the potential of incorporating nanomaterials into biological systems, they lack the precise integration of these ex vivo-synthesized nanomaterials into a target cell type or subcellular location, which is generally necessary for precisely modulating cellular functions within a multicellular organism [18–21].
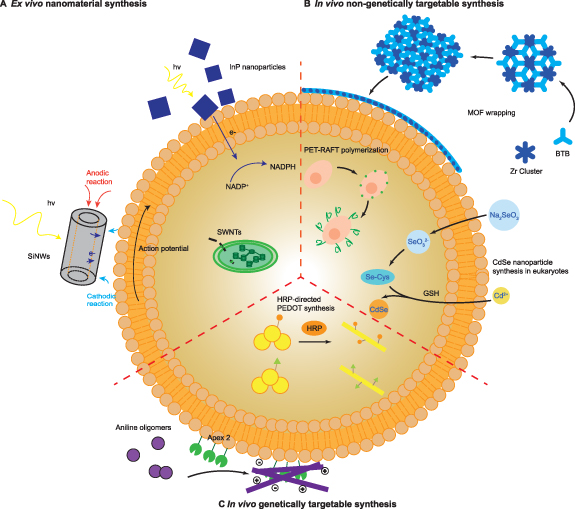
Figure 1. Nano-enabled synthetic biology. (A) Integration of ex vivo-synthesized nanomaterials with biology: silicon nanowires (SiNWs) can induce action potentials in neurons through a photoelectrochemical effect (left) [13]; single-walled carbon nanotubes (SWCNTs) can be incorporated into chloroplasts, enhancing photosynthesis (center) [16]; and InP nanoparticles can introduce photosynthetic capabilities to modified yeast (top) [9]. (B) In vivo non-genetically targetable synthesis: cells, including bacteria [22] and yeast [23], are wrapped with protective metal organic framework (MOF) coatings (top); cell-surface initiated polymerization can alter intercellular interactions (center) [24]; and CdSe nanoparticles can be synthesized in living plant cells (right) [25]. (C) In vivo genetically targetable synthesis: polythiophenes can be polymerized in vivo using horseradish peroxidase (center) [26], and conductive and insulating polymers can be deposited on cell membranes to modulate membrane capacitance using genetically-targeted peroxidases (bottom) [27].
Download figure:
Standard image High-resolution imageThus far, subcellular or cell-type specificity has largely been determined by innate properties of the cell type, organelle, or nanomaterial itself. One potential way of enabling the subcellular or anatomic specificity is direct synthesis of these nanomaterials in vivo (figures 1(b) and (c)). However, while there have been many efforts to synthesize nanomaterials in living systems, especially inorganic nanoparticles [28], relatively few of these nanomaterials have been used to augment functions directly in the organism in which they were produced. Therefore, there has been less motivation to target this in vivo nanomaterial synthesis to specific locations within tissues or cells. Some of the most notable examples of in vivo-synthesized nanobiohybrids include functionalizing the surface of individual cells including yeasts, bacteria, or stem cells with organic polymers [24, 29] or metal organic frameworks (MOFs) [22, 23, 30, 31] for purposes such as providing protection or influencing differentiation (figure 1(b)). In these contexts, concerns about intracellular toxicity or targeting specific cell-types are minimized. However, these remain significant obstacles to applying this strategy to more complex, multicellular organisms.
Recent advances in protein-directed nanomaterial synthesis may help overcome the challenge of precisely targeting nanomaterials to specific cellular locations within an organism by enabling genetic targeting of nanomaterial synthesis in vivo (figure 1(c)). Several enzymes [32, 33] and protein-based tags [34] with the capability of directly synthesizing or guiding the synthesis of nanomaterials have been described. By targeting these tags to certain subcellular destinations with genetic manipulation, much finer spatial control over the integration of these nanomaterials into biological systems can be achieved, promoting precise modulation and augmentation of cellular functions in more complex organisms. In a recent example, genetically-targeted peroxidases were used to deposit conductive and insulating polymers on the neuronal membrane, directly leading to increases and decreases in membrane capacitance and subsequent changes in neuron excitability, akin to changes in myelination [27]. As the number of enzymes or protein tags for directing nanomaterial synthesis grows, this genetically-targeted in vivo synthesis strategy can be readily applied to interfacing a variety of organisms with differing nanomaterials.
In this review, we discuss recent advances in engineering new biological functions at the cellular level, including examples integrating synthetic, ex vivo-synthesized nanomaterials, as well as those using in vivo synthetic approaches. In particular, we emphasize how cellular- or subcellular-level control can be achieved or applied. Though scarce, we also provide examples of genetically-targeted in situ nanomaterial synthesis. We envision that advances in biocompatible nanomaterial synthesis, particularly when targeted to specific organismal destinations, may open up new avenues for precisely controlled, nanomaterial-based synthetic biology.
2. Ex situ-synthesized nanomaterials
Integrating synthetic nanomaterials into living systems to produce nanobiohybrids provides researchers with a new way to control, study, and even enhance biological systems, complementary to more conventional genetic and chemical manipulations. In particular, interfacing organisms with nanomaterials allows us to introduce new functions or enhance existing biological functions in a manner not constrained by the central dogma. Although the biological component of nanobiohybrids may be any scale, including purified enzymes or organelles, here we focus on the cellular level, where manipulating and studying otherwise intact organisms are most relevant. Depending on the specific nanomaterial, organism, and desired outcome, the nanomaterial component may be prepared either in situ, directly in the organism of interest, or ex situ, for later integration with biological systems.
Despite an abundance of research in the biosynthesis of nanomaterials, ensuring that a nanomaterial with the desired physical properties can be synthesized specifically in the desired region of the organism in a biocompatible manner has limited the in situ synthetic approach to a few select examples. As such, many of the recent advances in nanobiohybrids research have taken the ex situ synthetic approach. Though in principle the nanomaterials used in this approach are limited only by their biocompatibility and synthetic accessibility, the most notable recent examples have primarily employed semiconducting nanomaterials to augment a variety of microbes, plants, and animal tissues. While applications have ranged from biosensing [35] to nanobiohybrid silk production [36, 37], recent efforts to interface ex situ-synthesized nanomaterials with living systems have largely concentrated on photosynthesis or controlling the electrophysiology of nerve and cardiac tissue.
2.1. Enhanced photosynthesis
At the microbial level, Yang et al have introduced photosynthetic capabilities to the nonphotosynthetic bacteria Sporomusa ovata and Moorella thermoacetica. In earlier work, the electrotrophic acetogen S. ovata was directly cultured on a TiO2-coated SiNW array [38]. S. ovata, when provided with reducing equivalents from the photoactive NW array, metabolized CO2 to acetate via the Wood-Ljungdahl pathway at a rate comparable to conventional gas-phase catalysis. Value-added chemicals including n-butanol, polyhydroxybutyrate, and several isoprenoids could then be synthesized from the acetate using genetically-modified Escherichia coli. Although this NW array confers some advantages, including allowing the obligate anaerobe to survive in aerobic atmospheres, the challenging synthesis of these NW arrays prompted Yang et al to instead use photosensitizing nanoparticles. In subsequent work, cadmium sulfide (CdS) nanoparticles were directly precipitated on the surface of M. thermoacetica [39]. Absorption of a photon generates an electron–hole pair, providing reducing equivalents for acetogenesis, while cysteine quenches the hole. Approximately 10% of the Acetyl-CoA generated by the Wood-Ljungdahl pathway was directed toward cell biomass, while the remaining 90% was converted to acetate, with no catabolic loss during dark cycles due to M. thermoacetica being unable to consume the acetate. More recently, the CdS nanoparticles were replaced by water-dispersed AuNCs (figures 2(a) and (b)), which were taken up by M. thermoacetica with 95% efficiency [17]. Intracellular AuNCs offered several advantages, being both more biocompatible than CdS nanoparticles and more efficient (figure 2(c)), bypassing the slow mass transport across the cell membrane.
Figure 2. (A) Depiction of the M. thermoacetica/AuNC hybrid system. The Au22(SG)18 nanoclusters were transported into M. thermoacetica (grey) during the culture process, forming M. thermoacetica/AuNCs with red emission (red). Light yellow spheres, Au atoms in the core; dark yellow, Au atoms in the staple motifs; red, S atoms in the shell. white, hydrogen; (B) schematic of bacterium showing the electron transfer and actetic acid generating pathways; (C) normalized photosynthetic production of acetic acid by M. thermoacetica, M. thermoacetica/AuNCs and M. thermoacetica–CdS PBSs under continuous low-intensity illumination and in dark conditions [17]; (D) assembly of S. cerevisiae–InP hybrids, in which InP nanoparticles were modified with polyphenol moieties, then assembled on the surface of yeast that were genetically engineered to produce modular inorganic-biological hybrids; (E) glucose consumption over 72 h culture in the S. cerevisiae–InP hybrid system; (F) specific shikimic acid yield based on consumed glucose and cell dry weight (CDW) [9]. (A)–(C): Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature: Nature Nanotechnology, [17], © 2018, The Author(s), under exclusive licence to Springer Nature Limited. (D)–(F): From [9]. Reprinted with permission from AAAS.
Download figure:
Standard image High-resolution imageComplementary to these efforts, Guo et al developed a genetically-modified Saccharomyces cerevisiae–InP biohybrid system capable of generating shikimic acid, a precursor for several drugs and fine chemicals [9] (figure 2(a)). Deleting ZWF1 disrupts the pentose-6-phosphate pathway, preventing the regeneration of NADPH. When illuminated, InP nanoparticles tethered to the surface of cells with a polyphenol-based method are able to provide electrons for the regeneration of NADPH from NADP+. Not only did this enable artificial control over central metabolic processes, but the illuminated biohybrids displayed superior shikimic acid production rates and decreased glucose consumption (figures 2(b) and (c)). In addition to introducing photosynthesis to nonphotosynthetic organisms, nanomaterials have recently been used to augment the natural photosynthesis of plants. Notably, Strano et al have integrated SWCNTs into chloroplasts to enhance photosynthesis by augmented photoabsorption [16]. Semiconducting SWCNTs can convert these absorbed photons to excitons that can then be transferred to the electron transport chain, increasing electron transport rates, unlike metallic nanotubes. These SWCNT biohybrids have also been developed for biosensing nitric oxide [16] and nitroaromatics [35].
2.2. Neuromodulation and cardiac modulation
In addition to modulating and enhancing primary metabolism, nanomaterials have also been used to control and augment the physiology of electrically active tissues in animals. When integrated with neurons and cardiac cells, semiconducting nanomaterials have been shown to directly change the cellular electrophysiological properties (e.g. cellular excitability) and physiological processes (e.g. growth and differentiation). In pioneering work, Ballerini et al demonstrated that neurons grown on conductive nanotubes display increased signaling activity [40]. Specifically, neural circuits grown on nanotubes exhibited a six-fold higher frequency of postsynaptic currents, although the cell type composition and intrinsic excitability of neurons were unchanged. It was later determined that the nanostructures were responsible for this effect, forming tight interactions with neuron membranes as revealed by electron microscopy [10] (figures 3(a) and (b)). Carbon nanotubes favored backpropagation of action potentials, inducing calcium electrogenesis and a subsequent after-potential depolarization that was observed in nanotube-interfaced neurons, but not those grown on planar conductive surfaces (figure 3(c)). More recently, single-layer graphene (SLG) was shown to also increase neural circuit activity by instead modifying intrinsic excitability [11] (figure 3(d)). The SLG was hypothesized to deplete potassium ions at the extracellular surface of the cell, consistent with the after-potential hyperpolarizations observed in both SLG neurons and those treated with K+ channel blockers (figure 3(e)).
Figure 3. (A) TEM sagittal section illustrates multi-wall carbon nanotubes–cell membrane contacts, indicated by black arrows [10]; (B) high-magnification micrographs from a section consecutive to (A); (C) the nanotube induced significantly larger after-potential depolarization (ADP) following a spike-train; (D) single-layer graphene (SLG) modulates neuronal communication; (E) depiction of the local amount of potassium ions depletion in the space between the cell membrane and the SLG surface (membrane/surface cleft) due to graphene trapping as function of cleft thickness. The light green region shows the extrapolated K+ depletion values (red line) within the range of the estimated cleft dimensions [11]; (F) nanowired (NW) three-dimensional cardiac patches. Pristine alginate (Alg) or Alg–NW composites as the culturing environment for isolated cardiomyocytes. Cardiac cells: (red), alginate pore walls: (blue), gold nanowires: (yellow); (G) cardiomyocytes cultured in Alg scaffolds (top) form small beating clusters, but synchronously beating cardiomyocytes in Alg–NW composites (bottom) have the potential to form organized cardiac-like tissue. Colors, contour lines and arrows represent the spatial and temporal evolution of the signal maximum; (I) calcium transients were observed at all points; (H) sites monitored in an Alg–NW scaffold. The stimulation point was 2 mm diagonally to the lower left of point I. The white arrow represents the direction of propagation; (K) calcium transients were only observed at the stimulation point in the unmodified scaffold. F/F0 refers to measured fluorescence normalized to background fluorescence; (J) sites monitored in pristine scaffold, where site I is the stimulation point [12]. (A)–(C): Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature: Nature Nanotechnology, [10], © 2008, Nature Publishing Group. (E): Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature: Nature Nanotechnology, [11], © 2018, The Author(s). (F)–(K): Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature: Nature Nanotechnology, [12], © 2011, Nature Publishing Group.
Download figure:
Standard image High-resolution imageThese same principles have been applied to modifying cardiac electrophysiology. Notably, Dvir et al incorporated gold NWs into alginate-based scaffolds for cardiac tissue engineering [12] (figure 3(f)). Cardiomyocytes and fibroblasts seeded into the matrix exhibited enhanced electrical and mechanical coupling, even before electrical stimulation (figure 3(g)). Calcium imaging revealed that the alginate-NW scaffold promoted synchronized contraction throughout the entire tissue upon electrical stimulation (figures 3(h) and (i)), unlike the traditional scaffolds (figures 3(j) and (k)).
In addition to modulating electrophysiology by implantation alone, these nanobiohybrid systems have also been paired with external stimuli, including light, magnetic fields, and ultrasound to introduce a greater degree of spatiotemporal control, akin to optogenetics [18–20]. In an early success, Huang et al managed to control ion channels with magnetic fields by exploiting the magnetothermal effect of superparamagnetic ferrite nanoparticles targeted to cells expressing the temperature-sensitive ion channel TRPV1 [41]. When later demonstrated in vivo, magnetic fields were able to stimulate the ventral tegmental area of mice overexpressing TRPV1 up to one month after initial injection of magnetic nanoparticles, without the glial activation and macrophage accumulation associated with macroscopic implants [42]. While this sort of genetic control can lend a greater degree of cell-type specificity to these systems, non-genetically targeted methods have traditionally garnered more interest due to the historic difficulty of genetic manipulation and the ethical concerns of genetic modification in humans.
This sort of remote control has been accomplished without genetic manipulations using piezoelectric nanoparticles coupled with ultrasound stimulation [43], but most recent examples have exploited the photothermal and/or photoelectric effect of nanomaterials to afford optical control. By coating gold nanoparticles (AuNPs) with Ts1, which binds voltage-gated sodium channels, Carvalho-de-Souza et al were able to target AuNPs to neuronal membranes and stimulate action potentials with 532 nm light [14] (figures 4(a) and (b)). In this photothermal mechanism, similar to direct membrane heating with infrared light [44], albeit with faster kinetics [14], the nanoparticle-induced heating transiently changes membrane capacitance, depolarizing the membrane (figure 4(c)). In a photoelectrochemical approach, Tian et al have used coaxial SiNWs to photostimulate action potentials in primary dorsal root ganglion neurons [13] (figure 4(d)). Upon light stimulation, these p-type/intrinsic/n-type (PIN) SiNWs generate a photocurrent that can locally depolarize a neuron (figure 4(e)), with limited photothermal contributions. Likewise, organic semiconducting polymers have also been investigated as optoelectronic devices for neuromodulation in part due to their favorable material properties, synthetic ease, and biocompatibility. Notably, Lanzani et al have used poly(3-hexyl)thiophene nanoparticles (P3HT-NPs) as light transducers in Hydra vulgaris, stimulating not only contraction behavior, but also up-regulating opsin-like gene transcription [15] (figures 4(f) and (g)). Taking a different approach, Głowacki et al engineered semiconductor quinacridone nanoparticles to photostimulate ion channels, likely through both photothermal and photocapacitive effects [45].
Figure 4. (A) Experimental set-up: AuNP-Ts1 were perfused over a patch-clamped DRG neuron through one side of a theta capillary. After a sufficient optical response is observed, fresh buffer is perfused over the cell, which is able to wash away the unmodified AuNPs but unable to wash AuNP-Ts1 from the cell; (B) representative traces of current-clamped DRG cells firing action potentials in response to two different stimuli: a 300 pA, 1 ms current injection (left side, blue bars) and a 174 mW, 1 ms 532 nm laser pulse (right side, green bars). As with non-functionalized AuNPs, DRG neurons initially respond only to electrical stimuli (blue bars: 500 pA, 1 ms), but addition of AuNP-Ts1 sensitizes them to optical stimuli (green bars: 174 mW, 532 nm, 1 ms). Here, however, washing does not quickly eliminate optical excitability; (C) top: temperatures of pure lipid bilayers, measured with a micropipette at a short distance above the membrane, continue to increase long after the end of the laser pulses; bottom: after rectification and filtering, the changes in capacitance (verified by the phase shift in the impedance analysis) are made clear. Capacitance increases linearly during the laser pulses, then decays back to baseline after a delay (or even small continued increase) that depends nonlinearly on laser pulse duration. Capacitance traces are averages of two acquisitions. In all traces, green arrows show the starts of optical stimuli [14]; (D) HAADF STEM image of PIN-SiNW (left) with p-type core outlined by white dotted line; (E) patch-clamp electrophysiology current-clamp trace of membrane voltage in a DRG neuron stimulated by injected current (blue pulse) and a laser pulse (green bar). Two conditions are displayed: laser only with no SiNW (left) and PINSiNW (right)[13]; (F) mechanisms underlying photoexcitation of P3HT-NPs. Top: Upon visible light illumination, primary photoexcitation states S1 are created within the polymer NPs, which can give rise to the creation of polaronic charged states P± or to nonradiative release of the excess energy ΔE. Bottom: different decay paths of the NP photoexcited states are shown: (a) photothermal excitation and heat release, (b) photoelectrochemical oxidation processes, and (c) electrical polarization; (G) average contraction scores and SD resulting from behavioral analysis (n = 60 polyps). Gray boxes indicate the 3 min light illumination period; black curves show contraction behavior of untreated polyps; red and blue curves show the contraction behavior of polyps treated with P3HT-NPs for 2 or 24 h, respectively [15]. (A)–(C): Reprinted from [14], Copyright (2015), with permission from Elsevier. (D) and (E): Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature: Nature Nanotechnology, [13], © 2018, The Author(s).
Download figure:
Standard image High-resolution imageOptoelectronic manipulation has also been applied to cardiac tissue, using the same general principles. Analogous photostimulation of cardiomyocytes using AuNPs [46], and SiNWs [47, 48], both dispersible and matrix-embedded, has been established. Graphene-based biointerfaces both have also been used for optical stimulation of heart activity in zebrafish embryos [49]. In addition to directly modifying neuron and cardiac electrophysiology, sensory organs have also been enhanced with nanomaterials. In a recent example, Ma et al enabled near-infrared (NIR) vision in mice through retinal injection of upconverting nanoparticles that emit absorbed NIR light as a green light [50]. These upconverting nanoparticles were targeted to photoreceptors by coating the surface with the protein concanavalin A, which binds to the sugar residues of the photoreceptor outer segment. Mice were able to discriminate shapes in NIR, without affecting normal vision in the visible range.
3. In situ-synthesized nanomaterials
Despite these successful integrations of ex situ-synthesized nanomaterials with a variety of biological systems at the cellular level, interfacing multicellular organisms with nanomaterials in a cell- or tissue-type specific manner remains challenging. Doing so with exogenously generated nanomaterials generally requires either precise implantation or designing of nanomaterials that are only taken up by certain tissues, cells, or organelles. Challenges of direct implantation include potential damage to tissues with surgical intervention, immune rejection of implants, and poor spatial resolution on the single cell to subcellular level, especially in tissues where different cell types are intermixed, such as the brain [8, 21, 51]. Coating nanoparticles with a small molecule, protein, or antibody that binds a specific target expressed on the desired cell type offers one potential solution, as demonstrated by the magnetic nanoparticles designed for neuromodulation [41] or the sub-retinal upconverting nanoparticles [50]. However, there are still a number of challenges associated with this method, including the availability of suitable cell markers and ligands for each cell type and subcellular trafficking of the nanomaterial within the target cell. For instance, while initial experiments targeted magnetic nanoparticles to genetically-modified neural cell membranes using a streptavidin-biotin acceptor protein system [41], this had to be abandoned in the in vivo application due to undesired cell internalization and formation of protein coronas that reduced the targeting and magnetothermal effectiveness in vivo [42]. This corona formation affects a wide array of nanomaterials, and impacts blood circulation of nanomaterials throughout the system in addition to subcellular location [52–54].
Alternatively, nanomaterials can be targeted to different tissues, cells, or even subcellular locations based on inherent nanomaterial properties. While certain nanoparticles may be absorbed at different rates by different tissues, as is the case for P3HT nanoparticles in H. vulgaris [15], purposefully engineering such targeting remains difficult. Recently, Strano et al have determined rational design principles for targeting a variety of nanoparticles to different subcellular locations in plants based on the membrane lipid composition and the nanoparticle size and zeta potential [55], but examples in other organisms remain scarce. For these reasons, directly synthesizing nanomaterials in the system of interest is an appealing alternative to using ex situ-synthesized nanomaterials.
In situ nanomaterial synthesis circumvents many of the issues with ex situ-synthesized nanomaterials. In particular, this strategy could enable targeted synthesis of nanomaterials in only the particular region of interest, obviating the need for surgical intervention, precise mechanical control of implantation, or the targeting strategies discussed above. In general, nanomaterial synthesis can be directed to specific regions within an organism by either relying on the innate properties of differing cell types, or by genetically targeting the synthesis through specific protein tags or catalysts. Although the latter method would afford much greater control over the system on the cellular or even subcellular level, until recently, most attempts at in vivo nanomaterial biosynthesis have focused on the former. This is in part due to a focus on producing raw nanomaterials for subsequent purification, where precise targeting to certain tissues, cells, or subcellular locations would be unnecessary or inefficient.
In addition to the focus on bulk nanomaterial production, genetic manipulation of many organisms has, until recently, been considered challenging and undesirable [8], and genetic tags specifically for producing or directing the production of nanomaterials have been lacking. Besides the notable advances in gene delivery, several recent demonstrations of genetically-targetable in situ nanomaterial synthesis [26, 27, 34, 56] are beginning to make this strategy more viable. Taken together, this recent progress in nanomaterial biosynthesis will greatly advance our ability to precisely design, create, and modulate a larger array of multicellular nanobiohybrid systems.
3.1. Non-targeted nanomaterial synthesis
Outside the context of synthetic biology, nanomaterial biosynthesis has long been investigated for the large-scale production of nanomaterials for a variety of purposes, providing several advantages over conventional production techniques. In addition to providing an easily-scalable, environmentally friendly platform for nanomaterial synthesis, biological systems often exert greater control over the composition and morphology of nanomaterials, resulting in less variation in size, shape, and composition [28]. Additionally, these materials are often produced as hybrids with endogenous biopolymers that can improve downstream biocompatibility [57] and colloidal stability in aqueous solutions [58]. For these reasons, many reviews have been dedicated to describing the biosynthesis of nanomaterials in a wide variety of organisms [28, 57, 59, 60]. However, the focus has generally been on isolating and purifying the biogenic nanomaterials for applications requiring pure nanomaterials such as optoelectronics, catalysis, and nanomedicine. As such, attempts at interfacing these nanomaterials directly with the producing organism to modulate or otherwise study the organism have remained scarce.
Although Yang et al's manipulation of the prokaryote M. thermoacetica with in vivo-precipitated CdS nanoparticles is one of the more prominent implementations of in situ production of inorganic nanoparticles, the technique is perhaps most useful when applied to eukaryotic cells and organisms, in which cellular and subcellular specificity are most necessary. Because the inorganic nanoparticles produced by eukaryotes tend to be eco-friendly, versatile and low-toxic, and have been widely used in many fields, numerous efforts have been dedicated to synthesizing inorganic nanomaterials in eukaryotes [28]. For example, Tarafdar and Raliya reported the synthesis of Zn, Mg and Ti nanoparticles by culturing fungus with various precursor salts [61]. These nanoparticles were found to be surprisingly stable (90 days for Zn and Ti and 105 days for Mg) and have been applied successfully in medical and agricultural sectors [28]. Other examples include the usage of plants [62], yeast [25] and even mammalian cells [63], but as of yet, few of these materials have been used for directly studying or manipulating the producing organism.
Besides inorganic nanoparticles, polymers have served as a popular candidate for in vivo synthesis, as they can be carefully tuned to be compatible with different physiological environments and the functionalities can be easily manipulated. These outstanding properties make polymeric materials some of the most studied materials for nanobiohybrid systems. Multiple assembly strategies have been designed to introduce the polymers into organisms, including layer-by-layer (LbL) sequential deposition and grafting from approach, which offer researchers with different manners of manipulating the formation of polymers.
LbL assembly, or a 'grafting-to' strategy, is an approach to grow ultrathin coatings of polymers that adhere to different surfaces [64]. These coatings can associate with cells through a variety of interactions, including electrostatic interactions, hydrogen bonding, van der Waals forces and covalent bonding. This sequential deposition technique is very simple and adaptable, for almost any surface can be coated with polymers via LbL, thus making it very powerful in constructing systems for antibiotic drug delivery, surface-mediated drug release and controlled differentiation of stem cells [65].
In situ polymerization is another powerful way to produce polymers through a 'grafting-from' manner in living cells, offering potentially greater specificity and spatiotemporal control when integrating polymers with living systems. For instance, Choi et al developed a novel method of surface-initiated, activator regenerated by electron transfer, atom transfer radical polymerization (SI-ARGET ATRP) for polymer coating on yeast surfaces [29]. During the synthesis, special dopamine-based ATRP initiators were used for achieving uniform deposition of the initiators on intended cell surfaces based on coating properties of polydopamine that did not vary with different materials. At the same time, the radical-scavenging nature of polydopamine also kept the targeted cells healthy from detriment of radicals during polymerization. A similar synthetic strategy was applied by Jia et al when they were modifying the surfaces of live yeast and mammalian cells [24]. With the usage of photoinduced electron transfer-reversible addition fragmentation chain-transfer polymerization (PET-RAFT), they were able to produce polyethylene glycol-based acrylamides with narrow polydispersity (Mw/Mn < 1.3) in only 5 min. With this optimized reaction condition, they were able to carry out cytocompatible initial polymerization and controllable in situ chain extension on cell surfaces (figure 5(a)). This highly efficient polymer grafting approach could be easily expanded to a variety of functional polymers, paving ways for promising applications such as manipulation of intercellular interaction (figure 5(b)).
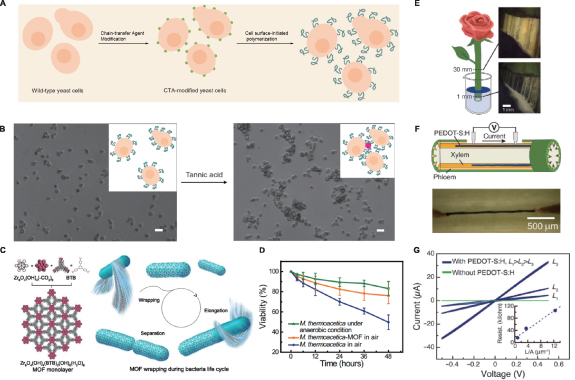
Figure 5. (A) PET-RAFT for polymer modification on cell surface; (B) manipulation of cellular phenotype by the grafted polymers. Tannic acid (TA), which can bind to PEG through hydrogen bonding interactions, is introduced to mediate aggregation of polymer-modified yeast cells [24]; (C) design and synthesis of the M. thermoacetica–MOF wrapping system; left: structural information of zirconium clusters and BTB; right: a demonstration of MOF wrapping, note this will not affect basic functions of cells; (D) cell population decay curves of M. thermoacetica and M. thermoacetica–MOF in air, and bare M. thermoacetica under anaerobic conditions [31]; (E) illustration of formed PEDOT-S:H wires in the xylem of a garden rose; (F) schematic of conductivity measurement using Au probes as contacts; (G) I–V characteristics of PEDOT-S xylem wires of different lengths. The inset shows resistance versus length/area and linear fit, yielding a conductivity of 0.13 S cm−1 [66]. (A) and (B): Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature: Nature Chemistry, [24], © 2017, Nature Publishing Group. (C) and (D): Reproduced with permission from [22]. (E)–(G): From [66]. Reprinted with permission from AAAS.
Download figure:
Standard image High-resolution imageMOFs serve as an emerging family of porous materials that are drawing significant interest as nanomaterials for synthetic biology. Given their unique chemical and physical properties, facile tunability in terms of the size and shape of pores, and the fact that they can be produced under physiological conditions with biocompatible starting materials, research regarding in vivo synthesis and potential synthetic biology applications of MOFs is developing rapidly. In their recent work, Falcaro et al fabricated zeolitic imidazolate framework-8 (ZIF-8) on the surface of living yeast, which they confirmed could serve as a 'selectively permeable exoskeleton' that protects cells from both large toxic proteins and relatively small chemicals like anti-fungal drug filipin [23]. Additionally, the presence of the MOFs coating could conserve cells in a man-made hibernation state where cell division was paused, and the removal of the ZIF-8 shells could restore the functionality of the cells. Besides directly growing cells in metal salt solutions, Yang et al have developed another method for MOF cell coating, in which a monolayer of MOF consisting of zirconium clusters and organic linkers was presynthesized before adding to the culture of bacteria [22] (figure 5(c)). This technology allowed the wrapping process to occur spontaneously, which eventually would form a protective shell on the surfaces that could reduce the death of anaerobic bacteria by fivefold in 21% O2 atmosphere (figure 5(d)). Moreover, cell elongation and separation could proceed even in the presence of MOF shells, and automatic wrapping occurred on the surface of the freshly divided cells.
As for in situ synthesis in multicellular organisms, there have been fewer reports, with the majority of work focusing on plants. Some of the most intriguing work involved direct incorporation of conductive polymers into living plants [66]. Stavrinidou et al immersed the fresh cross-section of a garden rose into an aqueous solution poly(3,4-ethylenedioxythiophene) (PEDOT), self-doped via a covalently attached anionic side group (termed PEDOT-S:H), to enable polymer deposition along the xylem channels (figure 5(e)). This formed conducting xylem wires that had long-range conductivity and could serve as components for in situ organic electrochemical transistors (figures 5(f) and (g)). In addition, the authors also explored the possibility of implanting electrodes in leaves. They applied vacuum infiltration to produce PEDOT:PSS (polystyrene sulfonate) combined with nanofibrillar cellulose in the apoplast of rose leaves, after which they observed field-induced electrochromic gradients, which opened up new avenues for designing novel organic electronics that could be synthesized and function in vivo. Their later study included a modified conjugated oligomer that could reach and form polymers in every part of the xylem vascular tissue of a rose, with which they could produce supercapacitors according to the architecture of plants [33].
With some inspiration from the earlier electronic plants, Liang et al explored the possibility of growing MOFs in plants. They demonstrated that various MOFs could be constructed inside diverse living plants, after which the fluorescent signal from the MOFs could be utilized for molecule sensing [67]. Their later work involved the introduction of modified MOFs species via root uptake, which could be used to detect specific toxic metal ions and organic molecules [68].
3.2. Genetically targetable nanomaterial synthesis
Despite these advances in in situ nanomaterial synthesis, few of these methods are able to target specific tissues or subcellular locations within organisms, limiting their applicability in complex multicellular organisms. Genetically targeting this in situ synthesis could provide the necessary spatial resolution and specificity for more advanced and less invasive manipulation of these systems. Direct examples of genetically-targeted inorganic nanomaterial synthesis are lacking in vivo, but efforts to functionalize metal-binding proteins such as ferritin [69] and metallothionein [70] are promising. Recently, Jiang et al reported a method of synthesizing AuNPs with uniform properties suitable for electron microscopy (EM) visualization on metallothionein-based tags [34]. While the method developed for EM staining is not wholly compatible with living tissue, similar protein tags could be developed to direct the synthesis of nanoparticles using the more biocompatible methods discussed above.
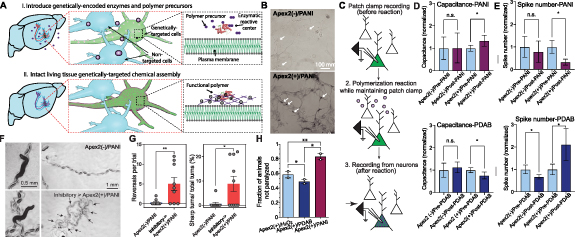
Perhaps more promising is the genetically directed synthesis of organic polymer-based nanomaterials. We recently reported the genetically targeted synthesis of conductive (polyaniline, PANI) and insulating (poly(3,3'-diaminobenzidine), PDAB) polymers on the membrane of neurons for directly modulating neuron excitability in vivo [27] (figure 6(a)). The engineered ascorbate peroxidase Apex2 was used to deposit both conductive and insulating polymers on cell membranes in the presence of hydrogen peroxide (figure 6(b)), directly manipulating the membrane conductivity. This polymerization led to increases and decreases in the membrane capacitance, decreasing and increasing excitability, respectively (figures 6(c) and (e)). In an in vivo model, the Apex2 was targeted to either GABAergic (inhibitory) or cholinergic (excitatory) motor neurons in C. elegans. Worms expressing Apex2 in inhibitory neurons, polymerized with PANI (inhibitory→Apex2(+)/PANI), exhibited sharper turns and increased reversal frequency, consistent with prior observations from analogous optogenetic manipulation of inhibitory neurons (figures 6(f) and (g)). Consistent with these results, excitatory→Apex2(+)/PANI worms became resistant to the acetylcholinesterase inhibitor aldicarb, while those polymerized with PDAB instead showed reduced aldicarb resistance, relative to non-transfected controls. In inhibitory→Apex2(+)/PANI worms, no aldicarb resistance was observed, demonstrating successful bidirectional control of both excitatory and inhibitory neurons with conductive and insulating polymers (figure 6(h)).
Figure 6. (A) Specific instantiation shown is enzyme/H2O2-catalyzed functional polymerization in brain. Blue indicates non-enzyme-targeted cells; (B) in situ genetically targeted synthesis and incorporation of conductive polymer. Shown are epifluorescence (YFP) and BF images of fixed rat hippocampal neurons. Arrows indicate individual neurons; (C) slice physiology workflow; (D) membrane capacitance and (E) current injection-evoked spikes before and after PANI polymerization [mean ± SEM, n = 7 Apex2(+) conditions, upper (D) n = 4 Apex2(−), upper (E) n = 3 Apex2(−); all individual cells were maintained in the whole-cell patch clamp configuration across pre-reaction and post-reaction time points for direct comparison; ratio-paired t tests:*P < 0.05]; all postconditions here and in bottom (D) and bottom (E) were normalized to corresponding preconditions for comparison; mean capacitance values were 20–45 pF. Bottom (D) membrane capacitance and bottom (E) current-injection-evoked spikes before and after PDAB polymerization; (F) representative bright-field images of Apex2(−)/PANI and inhibitory→ Apex2(+)/PANI worms (left) and their traces (right) after polymerization. Black arrows indicate where the worms took reversals; (G) left: summary of number of reversals for C. elegans during their movement. Right: the percentage of sharp bends (<90° bends) in total bends for C. elegans during movement; (H) summary of fraction-resistant C. elegans after 5 h in aldicarb resistance assay. From [27]. Reprinted with permission from AAAS.
Download figure:
Standard image High-resolution imageWhile this remains the clearest example of directly modulating eukaryotes with genetically targetable polymerization in vivo, the breadth of work on enzymatic polymerization provides many opportunities for developing genetic tags for in situ polymer synthesis. In particular, oxidases such as peroxidases and bilirubin oxidase have been used to synthesize a variety of functional polymers, including not only PANI [32, 71, 72] and PDAB [73], but also poly(methyl methacrylate) [74], polystyrene [75], and various functional polyphenols [76–80]. Recently, Stavrinidou et al have successfully synthesized polythiophenes with good conductive properties in vivo using horseradish peroxidase [26, 56]. Although these studies used an exogenous source of horseradish peroxidase, the physiological conditions used during polymerization and the cell permeability of the oligomers used suggest these methods could be used with endogenously expressed peroxidases, allowing this strategy to be adapted to a variety of systems.
4. Conclusion
Recent advances in the development of biocompatible functional nanomaterials have enabled a new paradigm of synthetic biology in which these nanomaterials, when integrated into living organisms, can augment or even create new biological functions. These advances have been bolstered by investigating both functional nanomaterials previously unexplored in a biological context, as well as new synthetic strategies. In particular, the increasing use of biocompatible, in vivo synthesis of functional materials promises unprecedented cellular or subcellular specificity. By enabling unparalleled anatomic- and cell-type-specific targetability of nanomaterials within living organisms, these techniques may significantly enhance our abilities to precisely construct and manipulate complex, multicellular nanobiohybrids. The rapid advent of nano-enabled synthetic biology has not only offered mechanistic insights into the interactions between nanomaterials and cells, but also provides new ways of manipulating organisms to both study their innate biological activity and impart novel properties to meet societal needs.
Nevertheless, the potential of these nanobiohybrid systems is far from being fully realized. Even though much effort has been made to understand the interface between nanomaterials and living systems, many fundamental questions regarding potential limitations remain unanswered in these newer systems, including the off-target effects of nanomaterials on certain cellular functions, and potential long-term cytotoxicity [81–83]. The synthesis and targeting strategies for nanomaterials developed in these recent works could also be extended to different organisms or physiological environments in order to explore diverse avenues of synthetic biology. Besides those we have mentioned here, there are still few examples of directing in vivo nanomaterial synthesis with specific protein tags or catalysts, which we expect to significantly advance our ability to integrate nanomaterials into living systems with more precise spatial control. Applied to new biological systems, these advances will result in a deeper understanding of how to devise and modify novel nanomaterials, contributing to fundamental advances in both nanoscience and biology.
Acknowledgment
J L acknowledges the support from Harvard University's Dean's Competitive Fund for Promising Scholarship.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).