Abstract
Stably recording the electrical activity of the same neurons over the adult life of an animal is important to neuroscience research and biomedical applications. Current implantable devices cannot provide stable recording on this timescale. Here, we introduce a method to precisely implant electronics with an open, unfolded mesh structure across multiple brain regions in the mouse. The open mesh structure forms a stable interwoven structure with the neural network, preventing probe drifting and showing no immune response and neuron loss during the year-long implantation. Rigorous statistical analysis, visual stimulus-dependent measurement and unbiased, machine-learning-based analysis demonstrated that single-unit action potentials have been recorded from the same neurons of behaving mice in a very long-term stable manner. Leveraging this stable structure, we demonstrated that the same neurons can be recorded over the entire adult life of the mouse, revealing the aging-associated evolution of single-neuron activities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data analyzed during the current study are available at https://github.com/LiuLab-Bioelectronics-Harvard/SpikeStability. Raw data generated during the current study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Blackrock Research Central Software Suite 7.04 was used to acquire electrophysiology data, available at https://blackrockneurotech.com/research/support/software/. Leica Application Suite X software platform 3.5.5 was used to acquire fluorescence images. Psychtoolbox-3 was used for visual stimulation administration, available at http://psychtoolbox.org. All data analysis and visualization in this study are implemented based on Python 3.7, MATLAB 2021a, Origin 2020 and Image J 1.53k. The following packages and software were used: R 4.1.0, RStudio 1.4, Jupyter 1.0.0, Anaconda 4.10.3, WaveClus3 (https://github.com/csn-le/wave_clus), Leiden 0.8.2 (https://github.com/vtraag/leidenalg), UMAP 0.5.2 (https://github.com/lmcinnes/umap), AllenSDK 2.10.1 (https://github.com/AllenInstitute/AllenSDK), tensorflow 2.5 (https://www.tensorflow.org), Monocle3 (https://cole-trapnell-lab.github.io/monocle3), MountainSort 4 (https://github.com/flatironinstitute/mountainsort), SpikeInterface 0.93.0 (https://github.com/SpikeInterface), scikit-learn 0.24.2, matplotlib 3.5.1, seaborn 0.11.2, numpy 1.21.5, scipy 1.7.3, pandas 1.3.5, cmasher 1.6.3, isosplit5 0.1.3 and pickle5 0.0.12. Custom code used in this study has been deposited on GitHub (https://github.com/LiuLab-Bioelectronics-Harvard/SpikeStability) and Zenodo (https://doi.org/10.5281/zenodo.7504820).
Change history
21 April 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41593-023-01329-0
References
Gallego, J. A., Perich, M. G., Chowdhury, R. H., Solla, S. A. & Miller, L. E. Long-term stability of cortical population dynamics underlying consistent behavior. Nat. Neurosci. 23, 260–270 (2020).
Steinmetz, N. A. et al. Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
Schoonover, C. E., Ohashi, S. N., Axel, R. & Fink, A. J. P. Representational drift in primary olfactory cortex. Nature 594, 541–546 (2021).
Dhawale, A. K. et al. Automated long-term recording and analysis of neural activity in behaving animals. eLife 6, e27702 (2017).
Dhawale, A. K., Wolff, S. B. E., Ko, R. & Ölveczky, B. P. The basal ganglia control the detailed kinematics of learned motor skills. Nat. Neurosci. 24, 1256–1269 (2021).
Wang, M. et al. Neuronal basis of age-related working memory decline. Nature 476, 210–213 (2011).
Igarashi, K. M., Lu, L., Colgin, L. L., Moser, M.-B. & Moser, E. I. Coordination of entorhinal–hippocampal ensemble activity during associative learning. Nature 510, 143–147 (2014).
Grady, C. The cognitive neuroscience of ageing. Nat. Rev. Neurosci. 13, 491–505 (2012).
Meng, G. et al. High-throughput synapse-resolving two-photon fluorescence microendoscopy for deep-brain volumetric imaging in vivo. eLife 8, e40805 (2019).
Salatino, J. W., Ludwig, K. A., Kozai, T. D. Y. & Purcell, E. K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 1, 862–877 (2017).
Ji, N. The practical and fundamental limits of optical imaging in mammalian brains. Neuron 83, 1242–1245 (2014).
Yu, K. J. et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 15, 782–791 (2016).
Chiang, C.-H. et al. Development of a neural interface for high-definition, long-term recording in rodents and nonhuman primates. Sci. Transl. Med. 12, eaay4682 (2020).
Liu, Y. et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 3, 58–68 (2019).
Yin, R. et al. Soft transparent graphene contact lens electrodes for conformal full-cornea recording of electroretinogram. Nat. Commun. 9, 2334 (2018).
Song, E. et al. Flexible electronic/optoelectronic microsystems with scalable designs for chronic biointegration. Proc. Natl Acad. Sci. USA 116, 15398–15406 (2019).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
Yang, X. et al. Bioinspired neuron-like electronics. Nat. Mater. 18, 510–517 (2019).
Fu, T.-M. et al. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 13, 875–882 (2016).
Guan, S. et al. Elastocapillary self-assembled neurotassels for stable neural activity recordings. Sci. Adv. 5, eaav2842 (2019).
Kim, T.-i et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
He, F. et al. Multimodal mapping of neural activity and cerebral blood flow reveals long-lasting neurovascular dissociations after small-scale strokes. Sci. Adv. 6, eaba1933 (2020).
Sharp, A. A., Ortega, A. M., Restrepo, D., Curran-Everett, D. & Gall, K. In vivo penetration mechanics and mechanical properties of mouse brain tissue at micrometer scales. IEEE Trans. Biomed. Eng. 56, 45–53 (2008).
McInnes, L., Healy, J. & Melville, J. UMAP: uniform manifold approximation and projection for dimension reduction. Preprint available at arXiv https://doi.org/10.48550/arXiv.1802.03426 (2018).
Tolias, A. S. et al. Recording chronically from the same neurons in awake, behaving primates. J. Neurophysiol. 98, 3780–3790 (2007).
Chung, J. E. et al. A fully automated approach to spike sorting. Neuron 95, 1381–1394 (2017).
McMahon, D. B. T., Jones, A. P., Bondar, I. V. & Leopold, D. A. Face-selective neurons maintain consistent visual responses across months. Proc. Natl Acad. Sci. USA 111, 8251–8256 (2014).
Schmitzer-Torbert, N., Jackson, J., Henze, D., Harris, K. & Redish, A. D. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131, 1–11 (2005).
Nguyen, T. et al. Automatic spike sorting by unsupervised clustering with diffusion maps and silhouettes. Neurocomputing 153, 199–210 (2015).
Kozai, T. D. Y. et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 11, 1065–1073 (2012).
Lu, L. et al. Soft and MRI compatible neural electrodes from carbon nanotube fibers. Nano. Lett. 19, 1577–1586 (2019).
Rousche, P. J. et al. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 48, 361–371 (2001).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Jeong, J.-W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
Airaghi Leccardi, M. J. I., Vagni, P. & Ghezzi, D. Multilayer 3D electrodes for neural implants. J. Neural Eng. 16, 026013 (2019).
Barrese, J. C. et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 10, 066014 (2013).
Viswanathan, P. & Nieder, A. Visual receptive field heterogeneity and functional connectivity of adjacent neurons in primate frontoparietal association cortices. J. Neurosci. 37, 8919–8928 (2017).
Jia, X. et al. High-density extracellular probes reveal dendritic backpropagation and facilitate neuron classification. J. Neurophysiol. 121, 1831–1847 (2019).
Marks, T. D. & Goard, M. J. Stimulus-dependent representational drift in primary visual cortex. Nat. Commun. 12, 5169 (2021).
Lee, E. K. et al. Non-linear dimensionality reduction on extracellular waveforms reveals cell type diversity in premotor cortex. eLife 10, e67490 (2021).
Zhao, S. et al. Full activation pattern mapping by simultaneous deep brain stimulation and fMRI with graphene fiber electrodes. Nat. Commun. 11, 1788 (2020).
Hill, D. N., Mehta, S. B. & Kleinfeld, D. Quality metrics to accompany spike sorting of extracellular signals. J. Neurosci. 31, 8699–8705 (2011).
Siegle, J. H. et al. Survey of spiking in the mouse visual system reveals functional hierarchy. Nature 592, 86–92 (2021).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Grieves, R. M. et al. The place-cell representation of volumetric space in rats. Nat. Commun. 11, 789 (2020).
Zhao, S. et al. Graphene encapsulated copper microwires as highly MRI compatible neural electrodes. Nano Lett. 16, 7731–7738 (2016).
Buccino, A. P. et al. SpikeInterface, a unified framework for spike sorting. eLife 9, e61834 (2020).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9, 5233 (2019).
Acknowledgements
We acknowledge the discussion and assistance from all Liu Group members, J. Salant and Prof. B.P. Ölveczky. We acknowledge the support from the Harvard University School of Engineering and Applied Sciences Startup fund and the Harvard University Faculty of Arts and Sciences Dean’s Competitive Fund for Promising Scholarship. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank scidraw.io for illustrations.
Author information
Authors and Affiliations
Contributions
J. Liu and S.Z. conceived and designed the experiments. S.Z., R.L. and J. Lee fabricated and characterized the electrodes. S.Z. and Z.L. performed the brain implantation and in vivo recording and histology study. S.Z. and W.T. conducted visual stimulation experiments. S.Z., W.T., X.T., S.P. and S.G. conducted the data analysis. J. Liu, S.Z., X.T., W.T., S.P. and H.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.L. declares financial interests in Axoft, Inc. All other authors have no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Michael Okun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Monolithically integrated tissue-level flexible mesh electronics.
Schematics showing the stepwise fabrication. a, A Ni sacrificial layer (grey) was defined by photolithography and deposited through thermal evaporation on the Si/SiO2 wafer (purple). b, SU-8 2000.5 bottom passivation layer (red) was defined by photolithography. c, Cr/Au interconnects and Pt microelectrodes (yellow) were sequentially defined by photolithography and deposited through electron beam (e-beam) evaporation on the top of the SU-8 passivation layer. d, SU-8 2000.5 top passivation was defined by photolithography (red). e, SU-8 2025 anchor was defined by photolithography (cyan). f, Dextran sacrificial layer (pink) was spin coated. g, SU-8 2025 shuttle was defined by photolithography (navy). h-m, Optical images illustrating each step of the fabrication corresponding with (b-g), respectively. n, Schematics showing the cross-section of the monolithically integrated mesh electronics at the red and black dashed boxes highlighted regions in (m), respectively. o-q, Contact profilometer measurements of the open mesh structure in (j, red dashed line), anchor structure in (k, cyan dashed line), and shuttle structure in (m, blue dashed line). r, Statistical summary of the thickness of the open mesh, anchor, and shuttle layer structures (n = 5 independent mesh electronics. Data are presented as mean ± s.d.). s-x, Schematics illustrating the preparation and releasing of mesh electronics for implantation. Color scheme is the same as in a-g. s, Side view of as-made mesh electronics. t, Flat flexible cable (FFC) was bonded to I/O pads of mesh electronics. u, The implanted part of the device was immersed in the Ni etchant to release the mesh electronics from the substrate. During this process, the dextran layer underneath the mesh electronics is also dissolved. The anchor and I/O pads maintain the overall geometry and intact structure of mesh electronics with the polymer shuttle. v, Wafer dicing was used to remove the substrate beneath mesh electronics. w, Dip coating of biodegradable PEG solution fixed the mesh electronics to the polymer shuttle. x, Anchor was removed before implantation.
Extended Data Fig. 2 Stepwise implantation of tissue-level flexible mesh electronics by the integrated polymer shuttle.
a, Schematics showing the stepwise implantation process. The polymer shuttle integrated with mesh electronics was inserted by manipulator#1. Then, manipulator#2 was used to extract the polymer shuttle. b, Photograph of the freestanding integrated device with the polymer shuttle integrated with mesh electronics, ready for implantation. c-f, Photographs showing the stepwise implantation process. c, The manipulator#1 was manually controlled to insert the integrated device into the targeted region of the mouse brain. The three-dimensional (3D) coordinates of the device were measured during insertion to control the implantation depth and speed. d, After the device reached the target position, a small drop of glue was gently placed on the polymer shuttle. e, A customized plastic pole mounted on manipulator#2, which was connected with the stereotaxic frame, was manually controlled to approach the glue. After the glue was completely cured and the PEG layer between the mesh electronics and polymer shuttle was dissolved, sterile saline solution was applied to further dissolve the dextran layer that bonded the polymer shuttle with the silicon chip at the input/output (I/O) region of the mesh electronics. f, The polymer shuttle was then carefully retracted from the brain tissue using manipulator#2, leaving the ultra-flexible mesh electronics in the implantation sites. g-h, Yield of insertion (g) and extraction (h) of 16-channel and 32-channel mesh electronics with different speeds (P > 0.05 for all comparisons. NS, not significant, two-tailed t test, n = 3 independent researchers, each researcher with 5 insertion/extractions. Data represented as mean ± s.d.).
Extended Data Fig. 3 Time-dependent histology studies of brain tissue reaction.
a, Representative photograph of mesh electronics with high-density electrode arrays. b-f, 3D reconstructed confocal fluorescence images showing mesh electronics sustaining open mesh structures across multiple brain regions, which interpenetrate with neural networks and introduce minimal immune response at 6-week post-implantation. b, High-density mesh electronics implanted in the cortex. Inset: zoomed-in views of the regions highlighted by the yellow box. Green, purple, blue and red label neurons, astrocytes, nuclei and mesh electronics, respectively. c, 16-channel mesh electronics implanted in both cortex and hippocampus with a designed 30-degree angle corresponding to the dorsal-ventral direction. d, Zoomed-in view of the hippocampal region highlighted by the cyan box in (c). e-f, Neuron interpenetrating with electrodes and interconnects with subcellular feature size. Individual electrodes are highlighted by yellow dashed circles in (d) and (e), tissue slice was labeled for neurons (green), nuclei (blue) and mesh electronics (red). g-h, Representative immunofluorescence images of brain tissues at 2-week, 6-week, 12-week, and 1-year post-implantation with mesh (g) and thin-film (h) electronics for characterizing immune response. Mesh and thin-film electronics are implanted in the contralateral hemispheres of the same animals. Tissue slices were labeled for astrocytes (red), microglia (purple), neurons (green), and nuclei (blue). Time-dependent histology studies have been repeated on n = 5 independent samples for each time point, with statistical analyses shown in Fig. 2f-i.
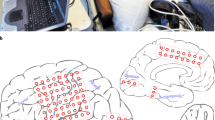
Extended Data Fig. 4 Characterization of single-unit recording quality of mesh electronics with tetrode-like electrode arrays.
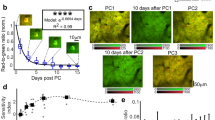
a, Bright-field (BF) microscopic image of representative mesh electronics with tetrode-like electrode arrays. Each mesh electronics consisted of five electrode arrays. Each array included six individually addressable electrodes for recording. b, Representative raw voltage traces showing spike signals recorded from two representative electrode arrays (orange and blue background labels, respectively) in an awake, head-fixed mouse at month 5. c, Zoomed-in view of the red arrow-highlighted region in (b). d, Representative single-unit waveforms were recorded simultaneously by multiple electrodes. Data represented as mean ± s.d. e, Inter-spike interval (ISI) distributions of representative single-unit spikes. f, Auto- and cross-correlograms (colored and black plots, respectively) of the representative single-units. g, Single-unit waveform centroids (n = 32 neurons) from a representative recording (centroid computed using spatial average across electrode positions weighted by the mean waveform amplitude at each electrode). h, Likelihood-ratio (L-ratio, 0.017 ± 0.013, mean ± s.d.) and Silhouette score (0.77 ± 0.09, mean ± s.d.) showing the clustering quality from identified neurons with multiple electrodes. n = 5 electrode arrays, and individual data points are overlaid. Box, 75% and 25% quantiles. Line, median. Whisker, the maxima/minima or to the median ± 1.5× IQR. The dashed lines are L-ratio of 0.05 (purple) and silhouette score of 0.5 (red), commonly taken as a threshold of high cluster quality. i, The probability density of refractory period violations (1.26% ± 0.93%, mean ± s.d., the refractory period violation is defined as an ISI < 1.5 ms, n = 32 neurons).
Extended Data Fig. 5 Clustering analysis demonstrating single-unit recording from a representative tetrode-like electrode array on mesh electronics.
a, Scatter plots showing peak-to-peak amplitudes of waveforms from all pairs of electrodes within an array. Identified clusters are plotted in colors. Multiple clusters are clearly evident representing the spikes of single units. b, The same waveform data projected onto the first principal component (PC1) axis of each electrode. The clusters can be distinguished and identified by multiple electrodes on both amplitude and PC1 space. that is, purple cluster: waveform recorded on electrode # 1 can be separated from waveforms on electrode # 2, 3, 4, 5, 6. Blue: electrode # 3 versus # 1, 2, 4, 5, 6; Cyan: electrode # 4 versus # 1, 2, 3, 5, 6; Green: electrode # 4 versus # 5, 6; Orange: electrode # 6 versus # 1, 2, 3, 4, 5. Yellow: electrode # 5 versus # 6.
Extended Data Fig. 6 Assessing the long-term single-unit recording stability using tetrode-like mesh electronics.
a, ISI distribution of 18 single neurons over the 7-month recording from a representative head-fixed, awake mouse. b, L-ratio (0.006 ± 0.006, mean ± s.d., n = 18 electrode arrays) and Silhouette score (0.84 ± 0.06, mean ± s.d., n = 18 electrode arrays) showing the clustering quality for the sorted single-unit action spikes over time. c-g, Firing rate (c), signal-to-noise ratio (SNR, red in e), amplitude (blue in e), peak-trough ratio (PT ratio, red in f), duration (blue in f), repolarization slope (red in g), and recovery slope (blue in g) of all the single-unit spikes as a function of time. Schematic (d) showing features extracted from the single-unit action potential waveform used for the analysis in e-g. Data represented as mean ± s.d., n = 92 neurons from 5 mice. Individual data points are overlaid. h, Single-neuron waveform centroids across a 7-month interval from a representative mouse. The centroid for each single unit was isolated on month 5 (blue circles) and months 6, 8, and 10 (red circles, rows 1-3, respectively). Grey circles indicate the positions of the mesh electrodes. i, Average displacement of single-neuron centroids from 5 mice between month 5 (blue circle, defined at origin) and months 6, 8, and 10 (red circles, rows 1-3, respectively). Grey contours indicate quintile boundaries of the distribution of centroid position displacement for the population (month 5 versus 6, n = 97 single units; month 5 versus 8, n = 96 single units; month 5 versus 10, n = 94 single units). j, Left, the cumulative distribution of within-unit centroid displacement (red) between month 5 and months 6, 8, and 10 (rows 1–3, respectively) and across-unit centroid displacement within a day (black) for 5 independent mice. Average on month 5 versus 6, within-unit = 2.86 μm (Q1 = 1.53 μm, Q3 = 3.76 μm), across-unit = 24.59 μm (Q1 = 17.46 μm, Q3 = 31.69μm); month 5 versus 8, within-unit = 2.35 μm (Q1 = 1.09 μm, Q3 = 3.17 μm), across-unit = 24.59 μm (Q1 = 17.66 μm, Q3 = 32.35 μm); month 5 versus 10, within-unit = 2.57 μm (Q1 = 1.22 μm, Q3 = 3.26 μm), across-unit = 24.68 μm (Q1 = 17.37 μm, Q3 = 32.53 μm). Two-sided Wilcoxon rank-sum test, P values are indicated in the graphs, n same as i. Inset at right, zoomed-in view to the 0 to 10 μm on x-axis.
Extended Data Fig. 7 Visual stimulus-evoked single-unit responses throughout 7-month recording.
a, b, Heat maps showing the responses to 18 static grating (a) and 12 dynamic grating (b) stimuli, 15 repeating trials for each recording with visual stimuli. Horizontal black bars indicate 1-s (a, static gratings) and 2-s (b, dynamic gratings) visual stimulus epochs. The activity of three single units across a 7-month interval to illustrate the stability of the same neurons and the diversity of neurons’ evoked response profiles to static and dynamic gratings. Heat maps for each month are normalized. c, Visual response of each neuron to static gratings in the first recording was matched with its own response and with the visual response of the physically closest other units in months 6, 8, and 10. d, Visual response of each neuron to dynamic gratings in the first recording was matched with its own response and with the visual response of the physically closest other units in months 6, 8, and 10.
Extended Data Fig. 8 Assessment of visual stimulus-dependent recording stability.
a-d Population summary comparing split-half correlations of static (a) and dynamic (c) gratings computed for trials drawn from the same neurons versus different neurons for each month recording. Dark blue (a, static gratings) and red (c, dynamic gratings): odd versus even trials from the same neurons; Light blue (a, static gratings) and red (c, dynamic gratings): odd versus even trials from all pairwise combinations of different neurons responding to the visual stimuli. Receiver operating characteristic (ROC) curve plots of false alarms (x-axis) against hits (y-axis) for static (b) and dynamic gratings (d).
Extended Data Fig. 9 Aged mice characterization.
a, Representative photograph showing the aged mouse of weight gain (18 months) with tissue-like mesh electronics implant (left) compared with the mature adult mouse (5 months, right). b, Zoomed-in view of the regions highlighted by white boxes in (a). c, Zoomed -in views of the thinning hair (white arrows) of aged mouse and glossy brown fur of mature adult mouse highlighted by red and blue boxes in (b). d-e, Representative photograph showing the barbering around eyes (d, red arrow), grey and thinning fur in the dorsal back skin (e, yellow arrows) of the aged mouse (18 months) with mesh electronics implant. f, Statistical analysis reveals that significantly increased gray hairs in dorsal back skin in old-aged mice (p = 0.013, *P < 0.05, two-tailed t test, n = 3 independent mice. Data are presented as mean ± s.d.).
Extended Data Fig. 10 Replicates of chronically stable tracking of neural activities of the same neurons throughout the entire adult life of mice.
a, Time evolution of single units clustered by Leiden clustering over the entire adult life of an additional representative head-fixed, awake mouse. The neural signals were recorded from the mature adult (5 months) to the aged (18 months) stage. The x- and y-axes denote the first and second uniform manifold approximation and projection (UMAP) dimensions, respectively, and the z-axis denotes mouse age in months. b, Time course analysis of the average waveforms of single-unit action potentials in the Leiden clustering results in (a). Data are represented as mean ± s.d.. c, Time evolution of ISI histograms of representative neurons from 5 months to 18 months. The x- and y-axes denote the time between subsequent action potentials of the firing neuron, and mouse age in months, respectively, and the z-axis denotes frequency. The bin size is 2 ms. Colors indicate individual neurons.
Supplementary information
Supplementary Information
Supplementary Text, Figs. 1–5, Tables 1–5 and Methods.
Supplementary Data 1
Statistical data for supplementary figures.
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Source Data Extended Data Fig. 9
Statistical Source Data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, S., Tang, X., Tian, W. et al. Tracking neural activity from the same cells during the entire adult life of mice. Nat Neurosci 26, 696–710 (2023). https://doi.org/10.1038/s41593-023-01267-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01267-x
This article is cited by
-
Graphene-integrated mesh electronics with converged multifunctionality for tracking multimodal excitation-contraction dynamics in cardiac microtissues
Nature Communications (2024)
-
Soft high-density neural probes enable stable single-neuron recordings
Nature Nanotechnology (2024)
-
A perspective on neuroethology: what the past teaches us about the future of neuroethology
Journal of Comparative Physiology A (2024)